Made in India surgical robot takes a giant leap for global triumph
 New Delhi: Perched on NASDAQ, SS Innovations, maker of ‘Made in India’ surgical robot, now takes a giant leap for its global triumph. With listing on USA’s major stock exchange, this Indian Medical Device MNC is poised to preside over all it surveys in the global surgical universe. This historic debut on NASDAQ is on top of 3,700+ surgeries by SSI Mantra, including 200+ robotic cardiac procedures — zero system-related complications reported. Add to it, surge of SS Innovations International’s revenue to $20.6 million in 2024 — a 3.5x increase over 2023 — with gross margin improving to 40.9%. Needless to say, with such top notch credentials, SS Innovations International is poised for global expansion with planned entry into Europe and the U.S.
New Delhi: Perched on NASDAQ, SS Innovations, maker of ‘Made in India’ surgical robot, now takes a giant leap for its global triumph. With listing on USA’s major stock exchange, this Indian Medical Device MNC is poised to preside over all it surveys in the global surgical universe. This historic debut on NASDAQ is on top of 3,700+ surgeries by SSI Mantra, including 200+ robotic cardiac procedures — zero system-related complications reported. Add to it, surge of SS Innovations International’s revenue to $20.6 million in 2024 — a 3.5x increase over 2023 — with gross margin improving to 40.9%. Needless to say, with such top notch credentials, SS Innovations International is poised for global expansion with planned entry into Europe and the U.S.
NASDAQ, exclusive club of stock exchange in USA, allowing any company to trade on its platform is not a mean achievement. It is an ultimate strong statement on the status and standard of a company. NASDAQ does not allow any company to be traded on its exchange. Only companies with a solid history and top notch management behind them get this privilege.
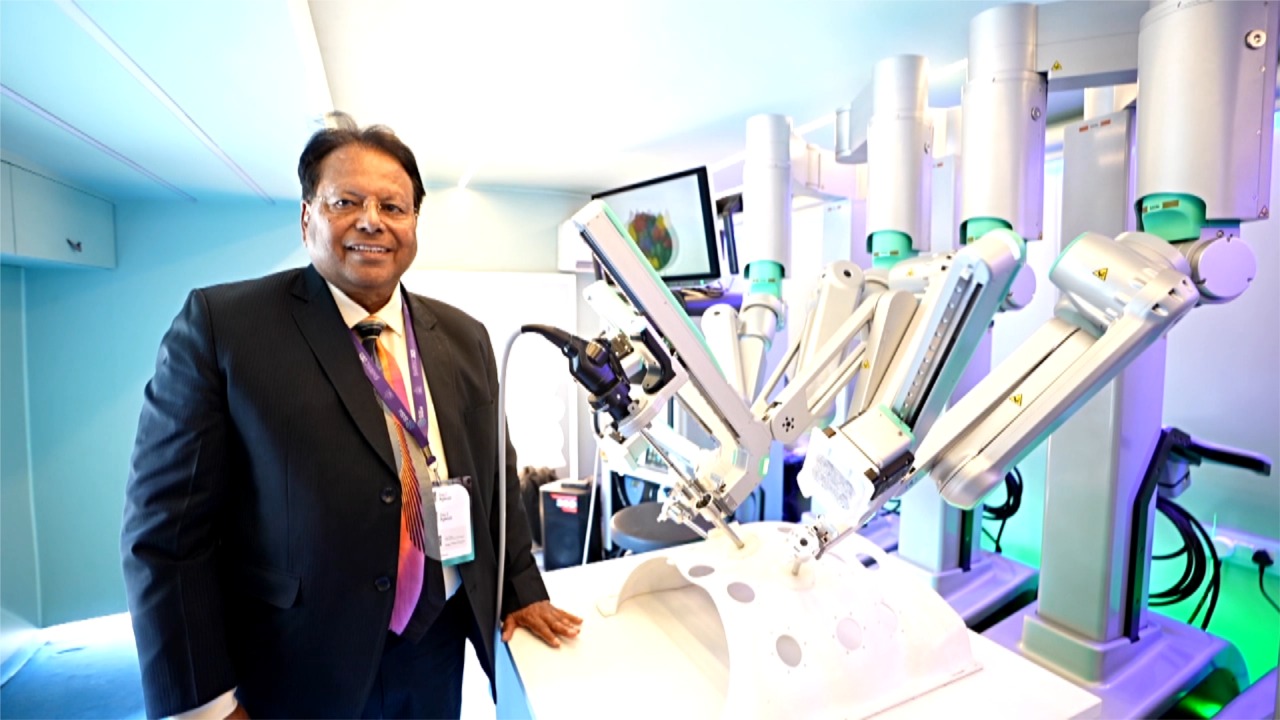
Listing of SS Innovations on NASDAQ is being looked at as a unprecedented Milestone of Indian Medical Technology on world stage. Trading has already commenced on April 25, 2025, with the company’s shares listed under the ticker symbol ‘SSII’. They say that this event is a prelude to India becoming global hub of medical devices. The script written by Dr Sudhir Srivastava, one of the greatest robotic surgeons of the world and indefatigable nationalist medical entrepreneur is an epic of Indian medtech talents. This achievement is propped by enviable financial foundations. SS Innovations International has reported an impressive financial growth for the year ending December 31, 2024, with revenues reaching $20.6 million — a 3.5-fold increase from the previous year’s revenue of $5.9 million. Gross margins also showed significant improvement, rising to 40.9% from 12.3% in 2023, highlighting the company’s robust financial performance and expanding market presence.
Furthermore, SS Innovations International has made remarkable progress with its clinically validated and patented SSI Mantra Surgical Robotic System, which has been installed in 80 hospitals across 75 locations in India, as well as expanding its footprint in countries including Nepal, Ecuador, Guatemala, the Philippines, Indonesia, Sri Lanka, and Ukraine.The SSI Mantra Surgical Robotic System is transforming access to advanced robotic surgery, offering a cost-effective solution to a broader patient population. With plans to expand into Europe and the United States, SSII is positioning itself as a global leader in the field of medical robotics, further solidifying India’s role in advancing healthcare technology worldwide.
Unable to contain happiness over such huge recognition Dr. Sudhir Srivastava, Founder, Chairman, and CEO, said, “Uplisting to NASDAQ marks an exciting milestone for SS Innovations International and reflects our team’s achievements in developing the world-class SSI Mantra Surgical Robotic System. This system offers top-tier quality, safety, efficiency, and affordability, enabling cutting-edge robotic surgical procedures for a wider range of patients globally. As a proud ‘Made in India for the world’ innovation, we are helping elevate India’s presence in the global healthcare landscape.”
“The timing of our uplisting coincides with our global expansion beyond India into multiple countries worldwide. We are pursuing the EU CE Mark and U.S. FDA approval for our SSI Mantra surgical robotic system, which we expect to receive in late 2025 and early 2026, respectively. We believe that this uplisting to NASDAQ will enhance market awareness of our growth story, improve transparency, expand our potential investor base, and ultimately increase share liquidity, all while showcasing India’s innovation on the global stage.” Dr. Srivastava added.
With over 3,700 surgeries performed using the SSI Mantra, including more than 200 robotic cardiac procedures, the system has demonstrated an impeccable safety record, with zero reported device- related mortality, injuries, or complications. Furthermore, SS Innovations became the first and only company in India to receive regulatory approval from the Central Drugs Standard Control Organization (CDSCO) for telesurgery and tele-proctoring procedures, marking a significant milestone in the company’s global expansion strategy.
Since receiving approval from India’s CDSCO, SS Innovations has successfully conducted 16 telesurgeries using the SSI Mantra Surgical Robotic System, including the world’s first robotic cardiac telesurgery and a landmark cardiac procedure performed across a distance of 2,000 kilometers — from North to South India. These groundbreaking surgeries, powered by the Made-in-India SSI Mantra Surgical Robot, not only underscore the company’s leadership in robotic and telesurgery but also highlight India’s emergence as a global innovator in advanced medical technologies.






