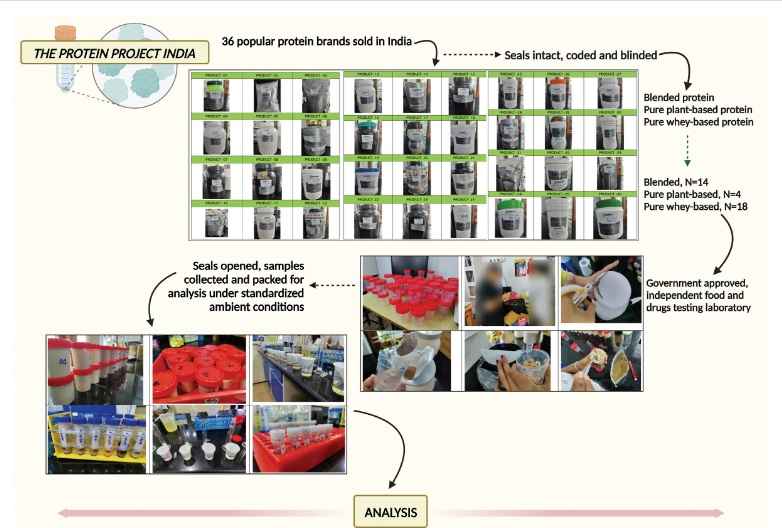
New Delhi: A first-of-its-kind observational analysis of the most popular protein powders sold and consumed in India has shown that the majority of these supplements falter on quality, labelling or advertised claims.
The findings of the analysis carried out on 36 different brands of protein powders, including those containing herbal and dietary supplements such as vitamins, minerals, and other natural or synthetic ingredients, were published in the peer-reviewed journal Medicine last week.
Protein supplements are extracts or concentrates of high protein foodstuff used for bodybuilding and as a dietary supplement to fulfil protein intake in a lean and pure source of proteins and amino acids (the building blocks of proteins).
The analysis showed that nearly 70 percent of the 36 supplements had inaccurate protein information, with some brands offering only half of what they claimed. Also, around 14 percent of samples contained harmful fungal aflatoxins, while 8 percent showed traces of pesticide residue.
Also, noted the authors — clinical researchers associated with Rajagiri Hospital in Kerala and a technology entrepreneur from the US — “most Indian-made herbal protein-based supplements are poor quality and contain liver toxic botanicals”.
“We demonstrate that the protein-based herbal and dietary supplement industry requires stringent scrutiny, regulation, and basic safety studies before being marketed,” the authors said.
Dr Cyriac Abby Philips, hepatologist from Rajagiri Hospital in Aluva in Kerala, the principal investigator of the self-funded study, told ThePrint that though there is published data from various research groups and clinical units across the world on organ damage, especially liver injury due to herbal and dietary supplements, there has been no proactive and prospective analysis of widely utilised supplements — especially protein-based — in published literature.
“There are occasional published reports that look at the quality of whey protein and amino acids analysis in protein supplements to identify amino acid spiking or ‘doping’ to falsely elevate protein content,” he said.
Philips added that one study also looked at how marketed protein supplements adhered to regulations with respect to quality — but much of this was done on protein supplements sold in the US and there were no such studies done from the Asia Pacific region.
“Our work sheds light on regulatory flaccidity, importance of consumers rights in being privy to transparency regarding choosing safe food or supplement options and general apathy of the medical community towards educating the public regarding food and diet supplements that are potentially beneficial versus potentially harmful,” he said.
ThePrint reached G. Kamala Vardhana Rao, chief executive at the Food Safety and Standards Authority of India (FSSAI), over calls, for comments on the findings in the paper but had received no response by the time of publication. This report will be updated if and when a response is received.
In response to a question in the Lok Sabha in August last year, Union Health Minister Mansukh Mandaviya had informed the Lower House that in 2022-23, as many as 38,053 civil cases and 4,817 criminal cases were lodged by the FSSAI for non-conforming food samples including protein powders and dietary supplements.







