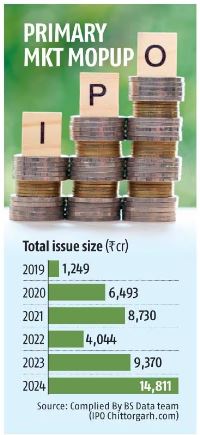
Nearly a decade after Johnson & Johnson’s subsidiary recalled its ‘ASR’ hip implants globally due to many patients requiring remedial surgeries, the Indian government wants the company to compensate local patients. J&J has already paid $2.5 billion in settlements in the US, while Indian victims may receive a minimum payout of Rs 20 lakh each after an arduous struggle for justice. ET traces the timeline of this controversy that has sparked fresh debates.
Joint Pain: How it intensified over the years
2003: J&J subsidiary DePuy Orthopaedics begins marketing ASR Hip Resurfacing System only outside the US
2004: DePuy launches ASR XL Acetabular System worldwide
2005: USFDA gives ASR XL marketing clearance following pre-market submission to demonstrate it’s at least as safe and effective as an already-present device
2006: DePuy International registers ASR XL Acetabular and ASR Hip Resurfacing systems for import and marketing in India
AUG 2009: USFDA sends DePuy a letter stating “significant additional data” would be required before final approval
DEC 2009: DePuy removes products from Australia upon its regulator’s intervention (J&J spokesperson claims it decided to discontinue the systems due to declining demand)
AUG 2010: DePuy initiates voluntary global recall after a UK study shows more patients required revision surgery than is normal for such devices
FEB 2010: India’s central drug regulator (CDSCO) gives DePuy’s fresh imports licence for implants on the basis of renewed registration
DEC 2010: In response to a query on the recall, DePuy tells CDSCO that 1,295 of 15,829 implants are successfully recalled, while 4,700 are already used in patients
SEPT 2011: CDSCO asks DePuy to share details such as recall procedure, reason and compensation details
OCT 2011: Maharashtra FDA asks CDSCO to take “corrective action”
DEC 2011: CDSCO asks DePuy for details of patients paid compensation again. DePuy says information is confi dential and only with surgeons and hospitals
JAN 2012: Maharashtra FDA tells CDSCO it has fi led an FIR against DePuy and asks it to cancel import licence for ASR implants
JULY 2012: CDSCO cancels import and marketing permissions “in public interest” and tells DePuy it failed to take proper measures to make patients aware implants were recalled due to “defectiveness”
NOV 2013: J&J announces $2.5-billion ASR settlement for around 8,000 patients in the US
DEC 2013: CDSCO issues a medical device alert to inform patients of problems related to implants
JAN–JUL 2014: DePuy informs CDSCO of “certain” patients who had undergone revision surgeries and reports deaths of four who had ASR implants
JULY 2014: Following news reports, CDSCO asks DePuy for details on action taken to identify patients implanted with ASR, related hospitals and compensation paid
DEC 2014: CDSCO and health ministry receive grievances on ASR implants and take it up with DePuy
FEB 2017: Health ministry constitutes an expert committee to look into the issue
FEB 2018: Expert committee submits its report, suggesting a base compensation of Rs 20 lakh per patient, among other recommendations
AUG 2018: Expert committee’s report is made public. Offi cials say ministry has accepted recommendations and is reaching out to states to trace affected patients
THE BARE BONES
93,000 patients received these implants globally, of which 4,700 were in India
J&J was able to recall only 1,295 of 15,829 implants from India in 2010
Only 1,032 patients with these implants in India have been traced
Over 250 Indian patients had to undergo repeat surgeries due to reasons like pain
At least four patients with these implants in India have died
J&J has “avoided” giving certain details related to these implants and the patients that received them, despite repeated requests, says a govt expert committee