Maryland: The FDA has approved a new type of stent designed specifically for infants and young children—including neonates born with congenital heart defects—that’s designed to expand in size as the child grows.
Renata Medical’s Minima implant aims to treat severely narrowed main arteries, including the aorta and the vessels leading to the lungs, where hampered blood flow can force the young heart to work much harder than necessary.
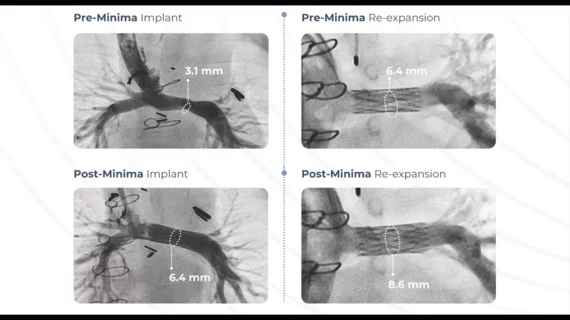
Just like stents for older cardiovascular patients, the Minima is delivered by a catheter through a minimally invasive procedure. The metal alloy implant is built out of long, thin struts, and starts off packaged down to less than 2 millimeters in diameter.
As the patient grows and the vessels become larger, they can return to the hospital for what the company described as a potentially one-day procedure to expand the stent further, up to 24 millimeters, and ensure that it continues to hold open the passageway.
“The approval of the Minima Stent is a huge milestone for our company, achieving the goal of providing the first stent designed and approved for small, growing children that are unfortunately some of the most vulnerable and overlooked patients,” Renata co-founder and CEO Dustin Armer said in a statement.
In a single-arm clinical trial reviewed by the FDA, the Minima stent was able to successfully open the blood vessels in 97.6% of patients, and 100% of the children treated went at least six months without major cardiovascular complications or new surgical interventions.
According to the company, 12 of the study’s 41 implanted patients, with a median age of 9 months, have since had their stents redilated—including one patient who has undergone the expansion procedure twice. Renata said all 13 operations were completed without any related adverse events.







