BOSTON: Servier has expanded its IDH drug armamentarium with the FDA approval for an IDH1/2 dual inhibitor. The approval represents the first major treatment advance in low-grade brain cancer in more than two decades.
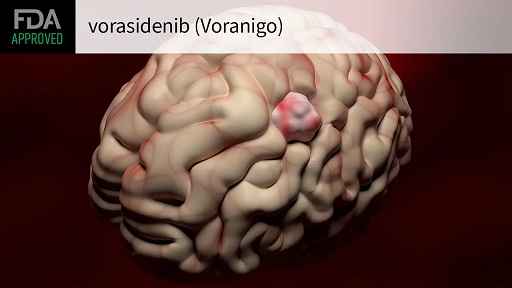
The new FDA approval is for Servier’s Voranigo, or vorasidenib, for adult and children ages 12 and older with low-grade glioma with a susceptible IDH1 or IDH2 mutation. As the FDA noted in its press release, this marks the first approval of a systemic therapy for this type of grade 2 IDH-mutant brain cancer, which includes oligodendrogliomas and astrocytomas.
Servier obtained Voranigo through its $2 billion acquisition of Agios Pharma’s oncology business in 2021. Royalty Pharma, which earlier this year acquired a stake in Agios’ royalty payments for Voranigo, has projected the drug could reach more than $1 billion in annual peak sales in the U.S.
Gliomas are the most common primary malignant brain tumors in adults, and IDH1 or IDH2 mutations are present in nearly all grade 2 diffuse gliomas in adults. By Royalty’s estimates, there are around 1,500 new diagnoses for these two IDH-mutant gliomas each year in the U.S.
Current therapeutic interventions for these patients are more than 20 years old and are all used off label, Islam Hassan, M.D., senior medical director at Servier, noted in an interview with Fierce Pharma ahead of Tuesday’s FDA decision.
Before Voranigo, Servier already has the IDH1 inhibitor Tibsovo and the IDH2 inhibitor Idhifa. Although doctors have been using the two meds off label because of the lack of treatment options, neither was approved for brain cancer.
Voranigo, for its part, was designed to penetrate the blood-brain barrier to be effective against brain tumors, Hassan explained.
In a phase 3 trial coded INDIGO, Voranigo significantly reduced the risk of tumor progression or death by 61% compared with placebo among patients with residual or recurrent grade 2 IDH-mutant glioma. These patients had undergone no previous treatment other than surgery.
In addition, Voranigo also showed a statistically significant benefit in delaying the need for the next treatment, slashing the risk for further treatment by 74% in the study.
The trial is ongoing to gather overall survival data. Still, Hassan argued that the progression-free survival data are just as important because these tumors will continue to grow and penetrate the brain and may develop into high-grade disease.
Besides the Voranigo monotherapy regimen, Servier has been evaluating the IDH inhibitor alongside Merck’s immunotherapy Keytruda in recurrent or progressive IDH1-mutant glioma.
While anti-PD-1 antibody drugs like Keytruda have been largely unsuccessful in brain tumors, Hassan pointed to a previous study in which patients received Voranigo and Keytruda before surgery. In tissues collected from surgery, Servier noticed some changes in the immune microenvironment that Hassan said warrants further development of this combination.
Servier is also testing Voranigo alongside the chemotherapy temozolomide in high-grade IDH-mutant glioma. As Hassan noted, these patients with grade 4 gliomas are typically excluded from other clinical trials.