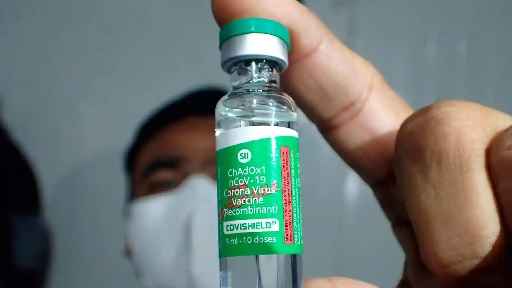
Mumbai: Serum Institute of India (SII), which produced AstraZeneca’s Covid vaccine under the brand name Covishield, on May 8 said it has stopped the manufacturing and supply of additional doses of the vaccine since December 2021.
Following the withdrawal of the Covid vaccine by AstraZeneca, SII said it acknowledges and fully understands the ongoing concerns around UK pharma major’s vaccine.
An SII spokesperson said it is committed to transparency and safety and had taken all the required steps to disclose the rare side effects of the vaccine.
On May 7, it was reported that the UK firm has announced that it will withdraw its Covid vaccine globally, citing an abundance of newer vaccine options available.
Additionally, AstraZeneca plans to revoke marketing authorisations for its vaccine, Vaxzevria, within Europe due to reduced demand and discontinuation of production and supply.
“As multiple, variant Covid-19 vaccines have since been developed there is a surplus of available updated vaccines,” the company said.
The UK drugmaker had recently admitted in a court that the vaccine can cause a rare side effect known as Thrombosis with Thrombocytopenia Syndrome (TTS).
The admission comes amid legal proceedings prompted by claims of serious health complications, including death, attributed to the vaccine. Lawyers representing numerous claimants argue that some cases could result in compensation payouts totaling up to 20 million pounds.







