 New Delhi: As ‘Make in India’ comes of age in medical device domain, it has started raising hackles of MNCs ruling the roost so far. Fear of losing market in the face of high quality medical devices made in India is driving them to resort to malicious tactics. Conspiracy is brewing to browbeat the resurgent Indian medical device companies making forays into outside progressive world.
New Delhi: As ‘Make in India’ comes of age in medical device domain, it has started raising hackles of MNCs ruling the roost so far. Fear of losing market in the face of high quality medical devices made in India is driving them to resort to malicious tactics. Conspiracy is brewing to browbeat the resurgent Indian medical device companies making forays into outside progressive world.
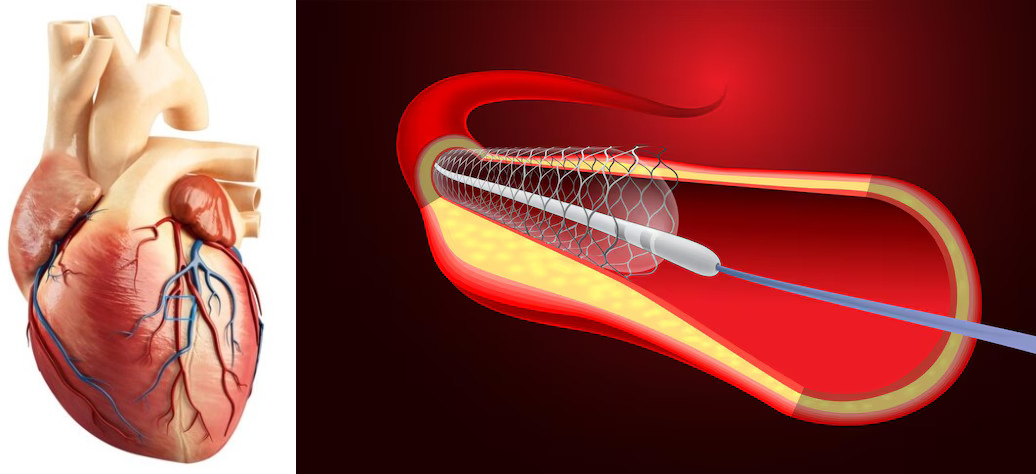
How a best in class coronary stent Supraflex Cruz was targeted and ambushed in Europe is a brazen case in point. Though Supraflex Cruz finally came out unscathed thanks to its superior quality and resilience, the purpose of recounting the whole gamut of gangism in play in an email to Union Government by Suprflex Cruz maker SMT is only to exemplify what might be on prowl in foreign turfs for even best of class Make in India medical devices.
This drug eluting stent manufactured by Sahajanand Medical Technologies Ltd (SMT), torch bearer of Make in India in medical device arena, is giving stent maker MNCs a run for their money. Though SMT showed exemplary resilience and frustrated the sinister design of powerful adversaries that be, thanks to superior quality of the product, the story of gangism that unfolded around Supraflex Cruz in Europe points to the fact that it could be jungle out there in foreign countries even for best medical devices made in India. The ominous design that tried to throttle this high quality stent speaks a volume about the rough and tumble that made in India medical devices might be pitted against.
Ganesh P Sabat , Chief Executive Officer of SMT, in an email to Dr Arunish Chawla, Secretary Pharmaceuticals gives a graphic account of how SMT stent found itself in a mesh of sinister design perpetrated by MNCs. The chronology enumerated by Mr Sabat clearly adds up to an ulterior game plan to frustrate Supraflex Cruz’s conquests as standard bearer of Make in India in European market. The way malicious tactics in Europe targeted a high quality medical device from SMT, it becomes crystal clear that Indian innovation might be under siege all over the world.
A proxy European law firm was harnessed by a competitor who was losing market share to SMT to bully Supraflex Cruz. This law firm started exerting undue pressure on the certification agency DNV. In a move that shocked many in the industry, DNV hastily suspended the CE mark for the Supraflex Cruz stent, a decision made without proper procedure and seemingly based on unfounded claims. The suspension threatened to undermine SMT’s hard-earned reputation and its foothold in the European market.
The harsh reality that emerges from this saga of shenanigans of medical device maker MNCs is that even the largest Indian manufacturers face such gangism and discrimination overseas. MNCs worst fear is real. Sooner or later, high quality medical devices made in India are poised to pip them to the post. Their market share is already losing its sheen. Unfortunately the engrained bias against the Drug Controller General of India and non acceptance of MNCs towards high end medical devices made by Indian manufacturers, despite robust clinical trials proving EU & USFDA products, Indian companies struggle to keep the Make In India clarion call a reality.
It is high time to call the bluff of MNCs restricting Indian companies from growing in Europe, even though the majority of EU nations including the big ones like UK, Germany, France , Switzerland, Scandinavian countries being heavily invested and influenced by China. There is need to adopt eye for an eye and a tooth for a tooth policy in no uncertain terms. We must sternly deal with merciless groupism which degrades Indian Drug Regulator, always proving that USFDA and CE are better yardsticks, and further gheraoing a reputed Indian company like SMT with false allegations, political pressure and fake claims.








