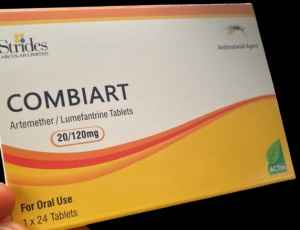
Lagos: The National Agency for Food and Drugs Administration and Control (NAFDAC) has raised the alarm about the circulation of counterfeit Combiart Dispersible Tablets (20/120mg) in Nigeria.
The product, supposedly manufactured by Strides Arcolab Limited, India, was discovered in the Federal Capital Territory (FCT) and Rivers State during surveillance operations conducted by NAFDAC’s Post Marketing Surveillance Directorate.
Laboratory analysis of the counterfeit tablets revealed they contained no Active Pharmaceutical Ingredients (APIs), rendering them ineffective for treating malaria. Furthermore, the product displayed discrepancies, including two different manufacturing and expiry dates, and an incorrect NAFDAC registration number not associated with the product.
Combiart, a combination of Artemether and Lumefantrine, is a widely used antimalarial medication for treating uncomplicated malaria caused by mosquito bites. However, counterfeit versions like this pose significant health risks, as they fail to meet safety, quality, and efficacy standards.
Counterfeit medications can lead to severe health consequences, including ineffective treatment, worsening conditions, and even death. NAFDAC has identified the counterfeit product with the following details:
Brand Name: Combiart Dispersible Tablet 20/120mg Generic Name: Artemether + Lumefantrine 20/120mg Batch No: 7225119 NAFDAC Reg No: A11-0299 Manufacturing Dates: June 2023 and February 2023 Expiry Dates: May 2026 and June 2026 Manufacturer: Strides Arcolab Limited, India
NAFDAC urges the public to remain vigilant and report any suspicious Combiart tablets to the nearest NAFDAC office or via its designated contact channels. This swift action is essential to protect public health and prevent further distribution of the counterfeit medication.






