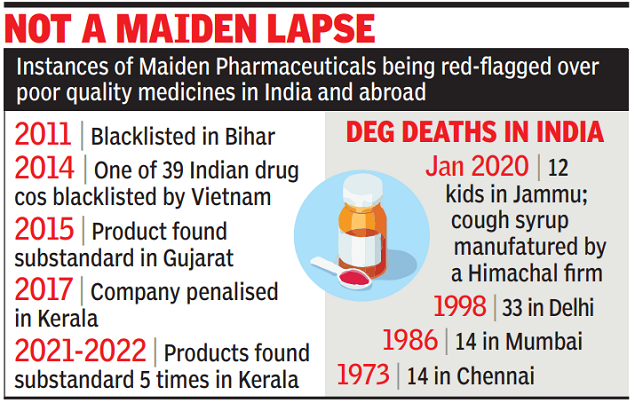
NEW DELHI: The Central Drug Standard Control Organisation (CDSCO) has been quick to assure people that the cough syrup which killed 66 children in Gambia was not being sold locally in India, but the same company, Maiden Pharmaceuticals, has been a repeat offender when it comes to producing substandard medicines in this country, with the earliest instance going back to 2011 when it was blacklisted in Bihar.
CDSCO has also stayed quiet on the fact that “unacceptable” amounts of diethylene glycol (DEG) and ethylene glycol, which is said to have led to the death of children in Gambia, has also killed children in Jammu in January 2020. DEG, which killed children in Jammu, was found in a cough syrup manufactured by a different company, M/s Digital Vision, based in Himachal Pradesh.
According to the Drugs and Cosmetics Act 1940, the punishment for manufacturing or trading spurious drugs that can cause death could include anything from 10 years to imprisonment for life, and a fine of Rs 10 lakh or three times the value of the drug confiscated. Till date, no one from Digital Vision has been penalized and all the accused are out on bail. Just as in the case of Maiden Pharmaceuticals, Digital Vision too was a repeat offender with seven instances between 2014 and 2019 of the company’s products being found “not of standard quality”.
Maiden Pharmaceuticals, established in 1990, has manufacturing units in Kundli and Panipat in Haryana and Solan in Himachal Pradesh, with the corporate office located in Pitampura, Delhi.
DEG deaths in India are not new. Other than the 12 children who died in Jammu in 2020, there were 33 deaths in Delhi in 1998, 14 deaths in Mumbai in 1986 and 14 deaths in Chennai in 1973. However, the CDSCO has stated that “exact one-to-one causal relation of death has not yet been provided by the WHO to the CDSCO”. DEG is used as a solvent in some drugs, but the permissible level is very low, just 0.1% to 2% in India.
TOI’s mail and messages to the CDSCO to seek its response on the issues raised here didn’t elicit any reply till the time of going to press. The story will be updated online if and when we get one.







