NEW DELHI: Have you ever got confused by similar looking or same sounding medicines? Here is some good news. Drug manufacturers may face strict action by the drug regulatory authority for identical sounding or similar looking brand names for drugs, in a move that could reinforce patient safety and mitigate the hazards posed by LASA (look-alike sound-alike) drugs.
India has been grappling with the issue of manufacturing and marketing of different drugs with the same brand name for long, a person in the know told ET.
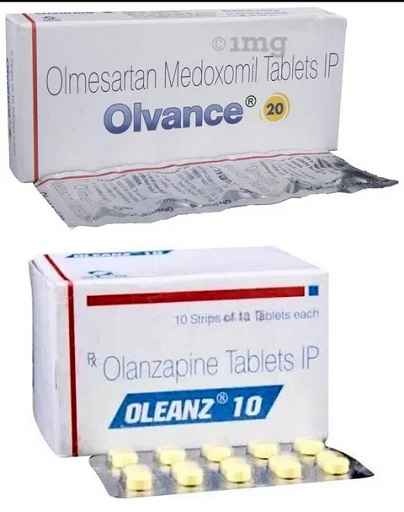
For example, the brand name, ‘Olvance’ for the antihypertensive drug, Olmesartan, and ‘Oleanz’, a brand of the antipsychotic drug, Olanzapine. Likewise, the brand, IMOX for amoxicillin tablets for humans, and INIMOX for a combination of amoxicillin and cloxacillin10 as an injection for veterinary use.
Earlier, in January India’s Drug Technical Advisory Board (DTAB), India’s apex drug advisory body, deliberated on the issue of same brand name (including look-alike and sound-alike ones) for different category of products and it suggested that to ensure patient safety, the manufacturing and marketing of different drugs with same brand name should “not be allowed.”
After that, the Director General of Health Services (DGHS) has written to the Controller General of Patents, Designs, and Trademarks under the ministry of commerce and industry seeking increased surveillance and monitoring of trademarks associated with pharmaceutical products to identify instances of similarity or confusion.
“It has come to our attention that various drugs, including those with sound alike and look alike names, are being produced and distributed under identical or similar trademarks. The situation not only creates confusion among healthcare professionals and patients but also increases the likelihood of medication errors, adverse drug reactions and other serious health consequences,” the letter said.
The DGHS’ letter sent in May was to ensure the integrity of pharmaceutical trademarks and protect public health by stricter implementation of trademark regulation, another person said.
The DGHS has asked the trademarks office to prioritise the issue and take immediate steps to strengthen trademark regulations for medicines.
“An urgent need has also been felt to take action by states against such manufacturers,” said a person in the know. The Drugs Consultative Committee (DCC) will deliberate and give its recommendations in the matter this month, he added.
A recent article published in Lancet, titled look-alike, sound-alike (LASA) drugs in India, had highlighted the issue which is pervasive in the country.
In the case of drugs with identical or similar brand names, there is no way a pharmacist could tell which drug the doctor had prescribed (in general, prescriptions in major parts of India only mention brand names with no mention of diagnosis or treatment protocol). Worse, there is no practical way of working out these ‘duplicates’ for any researcher, doctor, or pharmacist since there is no publicly accessible database that has all the drugs and the associated brands, said the Lancet.






