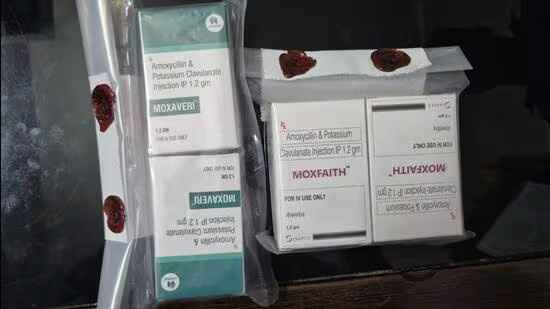
FDCA Raids Surat Firms Selling Fake Skin Products
Ahmedabad: Gujarat FDCA, in its third raid on fake allopathic drugs and cosmetics in the state in the last two months, busted three firms, all linked, in Surat for selling counterfeit…
Lilly Received Marketing Authorization For Tirzepatide In India
New Delhi: The popular weight loss and diabetes drug Tirzepatide — marketed as Mounjaro and Zepbound in the US — is likely to hit the markets in India though there has…
Mumbai: Drug Lord Reveals A Web Of Ops
Mumbai: Interrogation of drug lord Aliasgar Shirazi has revealed a web of operations spanning the courier and pharmaceutical sectors that served his illegal trafficking of psychotropic medicines to the United States…
Madras HC Sets Aside Ban On Online Sale Of Medicines
Chennai: In a relief for online pharmacies, including Tata 1mg, Practo, PharmEasy and NetMeds, the Madras High Court has reportedly set aside an earlier order by a single judge bench…
GB Nagar: Himachal Drug Makers Booked For Sub-Standard Medicines
NOIDA: The drugs control department of Gautam Budh Nagar district has registered a case against two Himachal Pradesh-located manufacturers/producers for allegedly making and selling “unfit” antibiotic medicines, officials aware of…
Glenmark Recalls 114 Batches Of Low Potassium Drug In The US Over Potentially Deadly Hyperkalemia Risk
MAHWAH, N.J. : While patients with low potassium often need medication to boost levels of the electrolyte in the body, too much of a good thing can cause serious health concerns.…
Maharashtra Drug Inspector Arrested For Taking Rs 1 Lakh Bribe In Palghar
Palghar: Maharashtra’s Palghar district witnessed a dramatic turn of events on Wednesday as the Anti-Corruption Bureau (ACB) arrested drug inspector Arti Shirish Kambli and Krishnakumar Asaram Tiwari for allegedly demanding…
DCA Seizes Overpriced, Misleading Claim Medicine
Hyderabad: Officials from the Telangana Drugs Control Administration (DCA) in separate raids in Sangareddy and Suryapet districts seized overpriced drugs and also medicines with misleading advertisements. During a special drive, DCA…
DoP Further Expands List Of Special Invitees Into Committee Constituted To Reform Pricing Framework
New Delhi: The Department of Pharmaceuticals (DoP) has once again expanded the list of special invitees for its Committee for reforms in the pricing framework for drugs and medical devices, formed…
Gujarat Receives Rs. 18,155 Crore Investment As Part Of VGGS 2023: Dr H G Koshia
Mumbai: Gujarat has received Rs. 18,155 crore investment as part of 167 MoUs signed in the Vibrant Global Gujarat Summit (VGGS) 2023, informed Dr H G Koshia, Gujarat Food and…