How did the dosing interval get longer?
Two studies were cited mainly for increasing the interval. In a Lancet study published earlier this year, efficacy result in phase-3 clinical trial of two doses of Oxford-AstraZeneca (Covishield equivalent) was 55.1% when the second dose was given in less than six weeks. This result was from a combined analysis of the participants from the UK, Brazil and South Africa. They indicated that the efficacy was 81.3% when the dosing interval stretched to 12 weeks or more. Next, AstraZeneca published interim results from phase-3 clinical trials in participants from the United States in March 2021, showing vaccine efficacy of 76% against symptomatic Covid-19 when the interval between doses was four weeks.
But supply shortage also played a part in India?
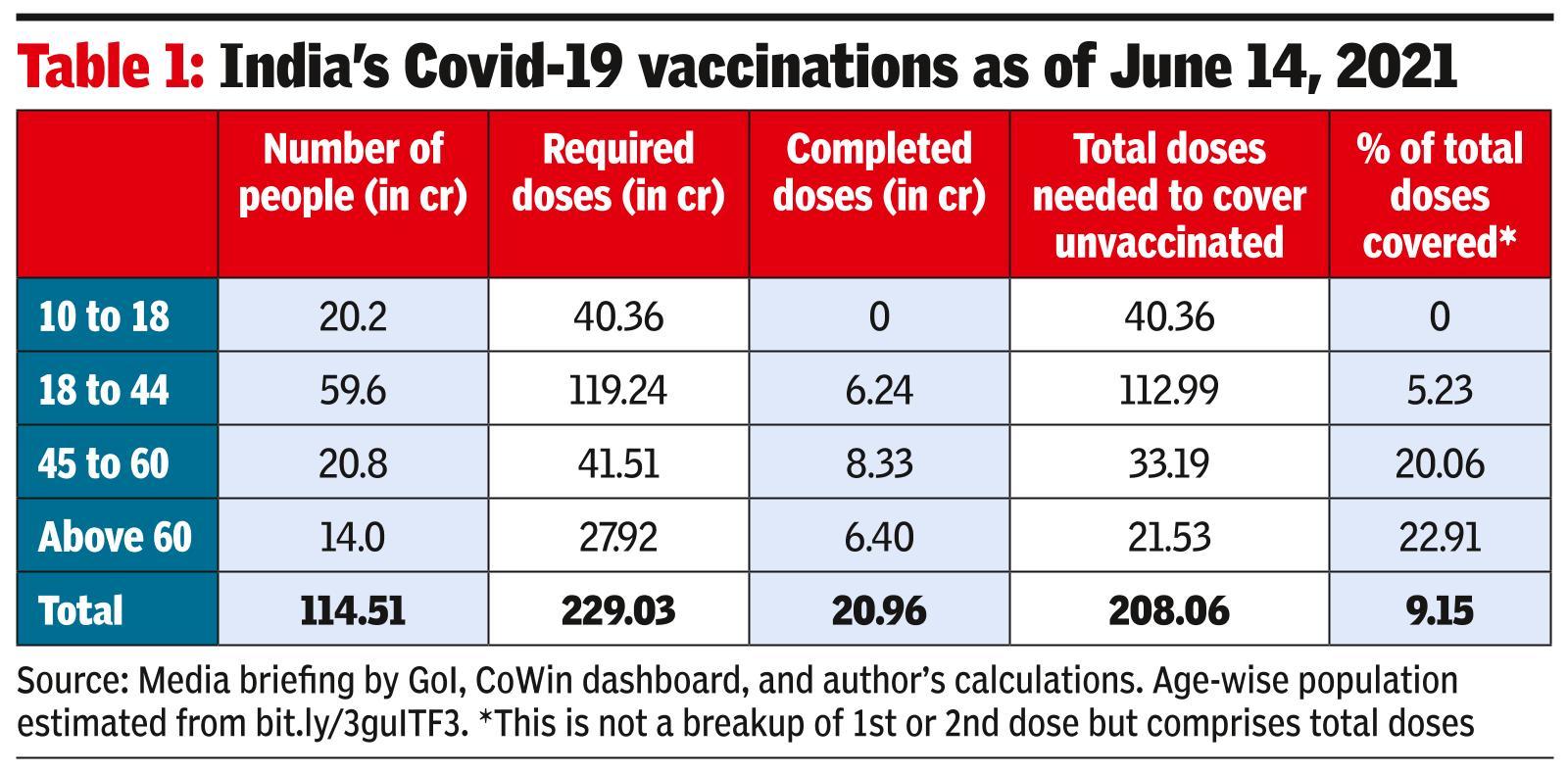
Yes. Even now, India’s vaccination effort is slow. After many months of coverage of the elderly (above 60 years), little over 22% are covered with either one dose or two doses. In the age group of 45-60, only 20% are similarly covered. Nearly 95% in the age group of 18-44 are yet to be vaccinated. We have only taken the baby steps so far. (See Table 1)
The Delta variant. Public Health England studies showed two doses of Oxford-AstraZeneca given at an eight-week interval are 92% effective against hospitalisation for the Delta variant. Now, UK data, which includes effects of a rapid vaccination programme, offers evidence that the second dose should be given quicker to get better protection against death and prevent hospitalisation.
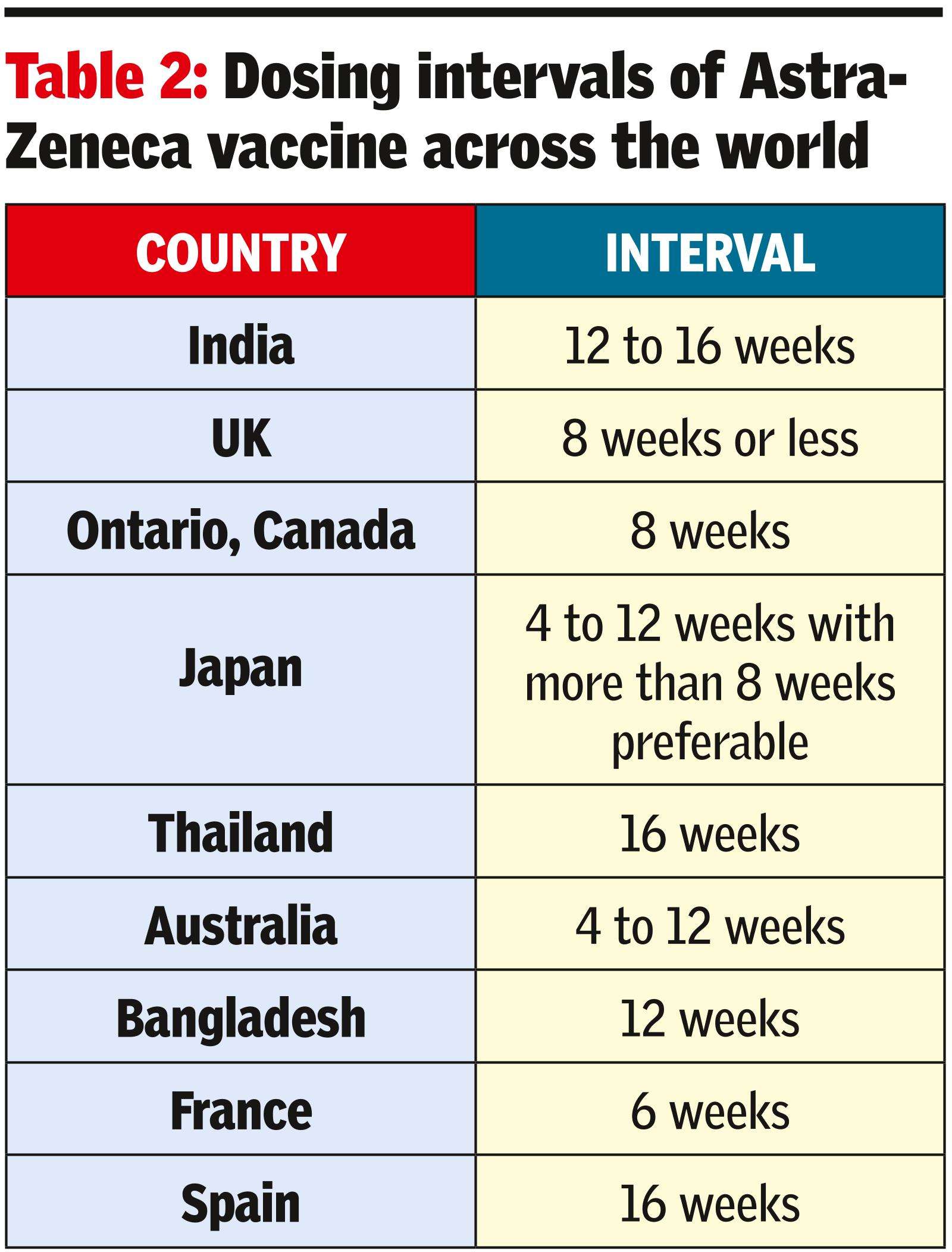
Which countries keep more than a 12-week interval between AstraZeneca shots?
India, Thailand and Spain. (See Table 2)
Are there other studies on Delta variant and AstraZeneca?
A recent study in The Lancet by Aziz Sheikh et al, suggests that the Delta variant of concern leads to higher rates of hospitalisation, especially in younger and more affluent groups. The study provides evidence that the two-shot Oxford-AstraZeneca vaccine effectively reduces the risk of SARS-CoV-2 infection and Covid-19 hospitalisation in people infected with the Delta variant.
What does this mean for India?
The Delta variant is the most dominant in India, resulting in several outbreaks in many parts. Like in the UK, other parts of the world are taking cognisance of findings regarding the Delta variant. Ontario in Canada has implemented a shorter dosage interval of eight weeks, reducing it from the earlier 12-week interval.
So, should GoI revise its policy now?
Yes. Vaccines are the best and the most important tool against preventing deaths due to Covid-19. When vaccines were in short supply for universal adult vaccination, extending the interval might have been necessary. However, given the evolving evidence that suggests two doses are effective against the Delta variant, GoI must revise its policy.
What should the interval be?
The interval should be reduced to four-eight weeks. This is the most effective way to ensure maximum protection against all existing and emerging variants. Reducing the interval is especially critical for persons with co-morbidities, irrespective of age.
The writer is Professor of Epidemiology at the Indian Institute of Public Health, Public Health Foundation of India, Bengaluru. Views are personal