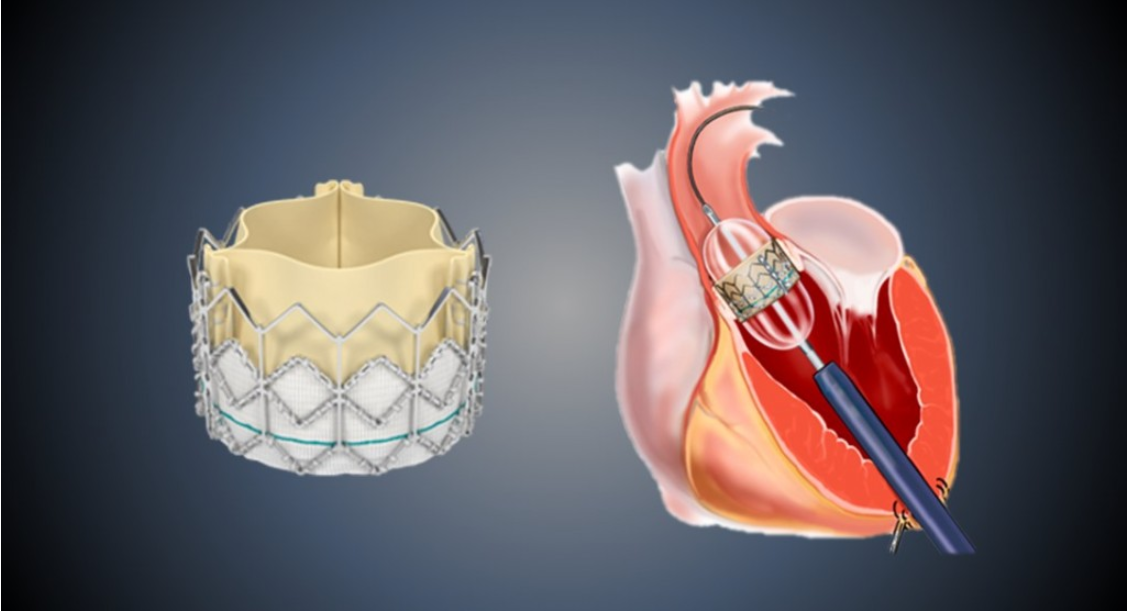
Zydus MedTech Private Limited, a wholly owned subsidiary of Zydus Lifesciences Limited specialising in medical technology development, has entered into a strategic partnership with Braile Biomédica Indústria, Comércio e Representações Ltda., (Braile Biomedica) – an innovative cardiovascular device manufacturer based in Brazil – to exclusively commercialise its Transcatheter Aortic Valve Implantation (TAVI) technology across Europe, India, and other select markets.
This agreement marks a significant step in Zydus MedTech’s strategic expansion into the fast-growing interventional cardiology segment. The global TAVI market, currently valued at over $6 billion, continues to witness strong growth driven by the increasing incidence of aortic stenosis and the rising demand for minimally invasive cardiac procedures.
Zydus MedTech, which is actively building its interventional cardiology portfolio, will introduce Braile Biomedica’s advanced balloon-expandable TAVI system to international markets, leveraging its commercial and regulatory expertise. Braile Biomedica – with a proven track record in cardiovascular innovation, particularly in Latin America – will manufacture and supply the product for these markets.
In addition to spearheading commercialisation, Zydus MedTech will also retain rights to manufacture select components of the TAVI system. This collaboration provides operational flexibility while laying the foundation for further product innovation and development.
The TAVI procedure is a recognised breakthrough in structural heart care, particularly for elderly patients or those at high surgical risk. By replacing the diseased aortic valve through a catheter-based approach – without the need for open-heart surgery – patients often experience significantly shorter recovery times and reduced procedural risks. The expansion of TAVI indications to all-risk patients has further accelerated its adoption.






