Ranchi : Patanjali’s case is going on in the Supreme Court for misleading people by showing misleading advertisements. But there are many other pharmaceutical companies in this category.
In fact, the Supreme Court has recently reprimanded Patanjali Ayurveda for misleading advertisements. Besides, the court has also ordered Baba Ramdev and Acharya Balakrishna to apologise. The case will be heard again on April 23.
Regarding another such case, Ranchi’s famous and young Dr. Anuj has posted a post on social media X. Which needs to be read and thought about and made aware.
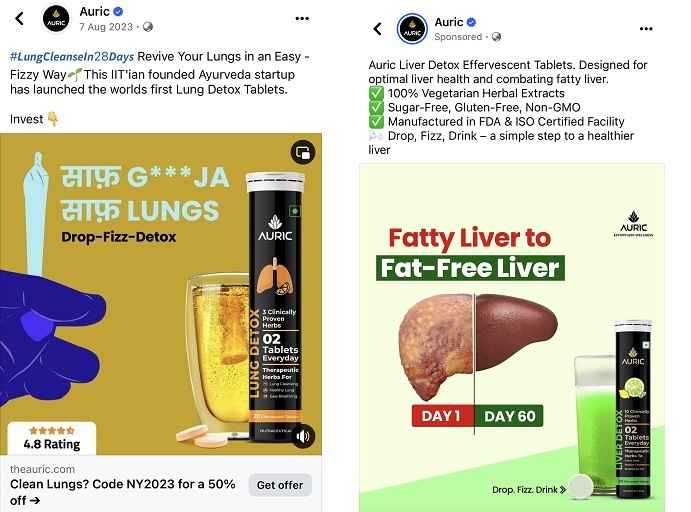
Dr. Anuj has written, there is a company named Auric which itself manufactures Ayurvedic nutraceutical products. It has claimed in the advertisement of this company that it completely cures fatty liver within 60 days. It also claims to completely cure lung problems caused by cigarettes and other intoxicants. It claims to call its products in Nutraceuticals category.
They say that they have launched these products in the market after doing a lot of research. If these are nutraceuticals then how are they claiming to cure the disease? Does this not fall in the category of misleading advertising?
When he was asked to share his research, he said that he has reviews on Amazon . Are Amazon and Google’s reviews research articles for them? What is the compulsion of FSSAI that all these misleading companies have been given free rein to play with people’s health?







