India‘s pharmaceutical companies will get an easy access to launch their products in the Australian market, with Therapeutic Goods Administration (TGA), the drug regulatory authority in Australia agreeing to expedite faster and easier approvals for India’s generic pharmaceutical products that have already been approved by developed countries such as the US, the UK, and European Union.
The Indian and Australian governments signed a pact last month aiming at strengthening ties in the pharma sector.
To ensure timely product approvals and boost demand for Indian pharmaceuticals in Australia, a seven-member delegation including officials from the department of commerce, drug regulator’s office, India Pharmaceutical Alliance and others had visited Australia in February this year.
Dinesh Dua, who was the chairman of Pharmaceutical Export Promotion Council (Pharmexcil), was also part of the delegation. Dua told ET that this will immensely help the pharmaceutical industry in getting early approvals to their products in Australia market.
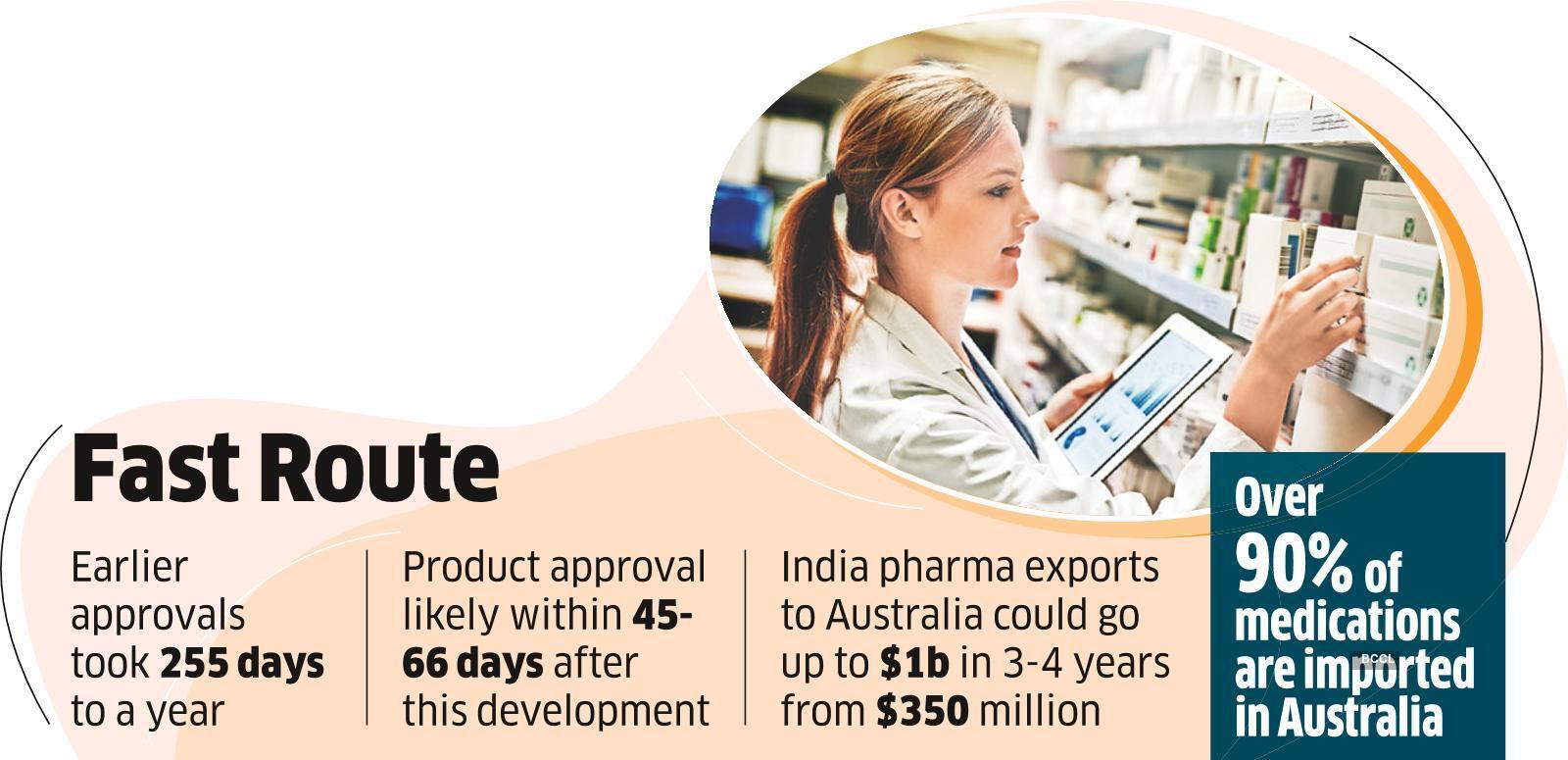
“Earlier the approvals would spill over from around 255 days to a year, but with this a product approval is expected to come within 45-66 days. If a report regarding a product/medicine is good and is certified by other regulators it will be granted approval in the Australian market without any inspections, resulting in easy access,” he said.
Dua said that the move will boost Indian drug makers’ exports to the southern nation. “India pharma exports to Australia to the tune of $350 million at present. This can go up to one billion dollars in about 3-4 years. India can be the biggest beneficiary of the export market,” Dua added.
Experts in the government said that pandemic has caused acute shortage of essential medicines and Indian pharma exporters can grab this opportunity to fill in the shortage and meet the demand. Australia depends a lot on Chinese APIs. India can be a partner with Australia for APIs and drug intermediates.
Over 90% of medications are imported in Australia which is at the end of a lengthy global supply chain, making it vulnerable to supply chain disruptions.
India plans to collaborate with Australia on drug repurposing, a method that allows current licensed drugs to be used for new medical conditions, reducing drug development time, pre-clinical trial time, and expenses significantly.