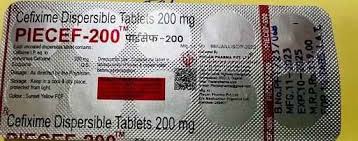
Jaipur: Four samples of cefixime antibiotics, produced by the same pharma company that was raided by FDCA in Gujarat, have failed the quality test in Rajasthan.
The seized samples of the antibiotic had no cefixime in them. Piecan Pharma, the drug manufacturer, was raided by FDCA in Gujarat during a crackdown on spurious drugs. The same drugs, also being sold in Rajasthan, were seized by FDCA and Rs 6 lakh worth of the spurious antibiotic was recovered. The state team has informed its Gujarat counterparts.
“The cefixime tablets manufactured by Piecan Pharma failed quality test. We found that it did not contain cefixime content in it,” said Ajay Phatak, drug controller (I), health department. Phatak said, “Spurious drugs racket was busted in Gujarat where action against Piecan Pharma was taken.”
The state’s FSDC also conducted searches. “We found these drugs stored at 13 places. We have seized the stock of these drugs which costs around Rs 6-lakh,” said Phatak.






