Another round of violation by the same company continues. The Drug Price Control Order 2013 being violated by same company repeatedly even after the reports of their violations are being published by “Medicare News” in their newspaper. It is matter of concern that the big companies have little fear of law as per survey conducted by the “Medicare News”. It noticed that the company “M/s Zydus Cadila Healthcare, M/s Sun Pharmaceutical and M/s Dr. Reddy’s” continues their march for violation of DPCO, 2013. It is matter of great concern that the price of the drugs which is of mass use has been fixed by the “NPPA” in exercise of the power given under Para 19. It is important to note that the power given under Para 19 are used only in the necessary situation and after analyzing the mass utilization of the drug. The power under Para 19 is purely on the basis of public interest. In our endeavor to highlight such violations we are giving below the price violations by the companies. It is important to note that same company was reported by the “Medicare News” finding them violating the DOCP 2013.
NPPA Vide Notification No. S.O. 1745(E) dated 10th July 2014 under Para 19 fixed the MRP for the drug “Carvedilol” 3.12mg at Rs. 2.69 per tablet. The aforesaid price has been fixed for M/s Zydus Cadila. The said company is selling the aforesaid formulation “Carvedilol” at Rs 3.55. Thus on the strip of ten tablets the overcharging amount comes to at Rs. 8.60 which comes to 31.97% of control price.
NPPA again Vide same Notification No. S.O. 1745(E) dated 10th July 2014 under Para 19 fixed the MRP for the drug “Carvedilol- 3.12mg” at Rs. 2.69 per tablet for M/s Sun Pharma. However said company Sun Pharma is marketing “Carvedilol- 3.12mg” under brand name “Cardivas 3.125mg” at Rs. 39/- for ten tablets against MRP of price of Rs. 26.90. This clearly shows the overcharging of Rs. 12.10 for ten tablets which constitutes overcharging of 45.33%.
In another case of overcharging that is reported by our special correspondent relates to the drug “Clopidogrel-300mg”. Vide Notification No. S.O. 1746(E) dated 10th July 2014 the NPPA has fixed the MRP of said formulation containing 300mg tablet at Rs. 36.67. It has been learnt that M/s Dr. Reddy’s is marketing said formulation of “Clopidogrel 300mg” under brand name “Plagril Gold 300mg” and said tablet is sold in the pack of two tablets at Rs. 103/-. Thus one tablet is sold at Rs. 51.50. There is clear overcharging of Rs. 14.93 for per tablet. This comes to overcharging of 40.82%.
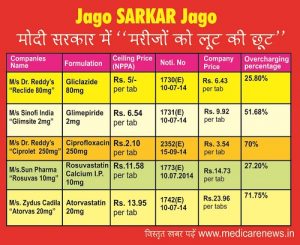
It is stated that in our previous issues we have covered the overcharging by various companies and list of such overcharging is increasing day by day. The following table will make it clear that how the price of a drug is recovered from the poor people by the companies even after the fixation of a price under Para 19 which can be used by the NPPA in emergent situations only.
| Companies Name | Formulation | Ceiling Price (NPPA) | Noti. No | Company Price | Overcharging percentage |
| M/s Dr. Reddy’s | Gliclazide 80mg | Rs. 5/- per tab | 1730(E) | Rs. 6.43 per tab | 25.80% |
| “Reclide 80mg” | 10-07-14 | ||||
| M/s Sinofi India“Glimsite 2mg” | Glimepiride 2mg | Rs. 6.54 per tab | 1731(E)10-07-14 | Rs. 9.92 per tab | 51.68% |
| M/s Dr. Reddy’s | Ciprofloxacin 250mg | Rs.2.10 per tab | 2352(E) | Rs. 3.54 per tab | 70% |
| “Ciprolet 250mg” | 15-09-14 | ||||
| M/s.Sun Pharma | Rosuvastatin Calcium I.P. 10mg | Rs.11.58 per tab | 1773(E)10.07.2014 | Rs.14.73 per tab | 27.20% |
| “Rosuvas 10mg” | |||||
| M/s. Zydus Cadila | Atorvastatin 20mg | Rs. 13.95 per tab | 1742(E)10-07-14 | Rs.23.96 per tabs | 71.75% |
he above table and the report carried on here above clearly shows that the overcharging is rampant one and regular feature. It is understood that once the emergent powers are invoked by the NPPA these companies have no right at all to sell the drug over and above price fixed by the NPPA. Even admixtures of any other ingredient or API will have to be considered as new drug and subject to the provisions of Para 15 and needs price approval.
It is seen that the NPPA is pending Crores of rupees in collecting the data relating to the overcharging and whereas the “Medicare News” is giving authenticated and verified data and figures free of costs but still the top bosses of NPPA are silent. It could be understood that top bosses are always busy in policy making or some other work but what about the mid level officers who are in charge of overcharging. The lapse of any action in NPPA is attributable to the corrupt practices in the organization and persisting inaction clearly shows that price regulator NPPA should be made corruption free.
The “Medicare News” will be coming out with some more violation by very these same companies after completion of investigation which is in process now.
“Jago Grahak Jago”
Mr. Vinay
Special Correspondent.








