Proteins are life’s engines, powering processes like muscle movement, vision, and chemical reactions. Their environments-water, lipid membranes, or other condensed phases-are critical to their function, shaping their structure and interactions.
Yet many modern protein-design methods, including AI-based tools, often ignore how these surroundings influence proteins. This gap limits our ability to create proteins with new functions, slowing progress in medicine and bioengineering.
One group of proteins working in such specialized environments are the membrane receptors, which act like biological “antennas”, sensing signals from the environment and triggering cellular responses.
Among proteins, the G-protein-coupled receptors (GPCRs), are central to how cells sense and respond to external stimuli. To carry out their signaling, GPCRs rely on a delicate interplay between structural stability, flexibility, and ligand binding, balancing acts that are often mediated by water. These collectively allow GPCRs to switch shape and communicate the signals they receive into the cell.
So crucial are these molecular gatekeepers for normal cellular function that around one-third of all drugs on the market target them. But GPCRs are also at the forefront of protein engineering, with efforts made to tweak these receptors to boost drug efficacy, develop novel disease treatments, and even to repurpose them as biosensors in synthetic biology.
The catch? GPCRs are incredibly complex, and their delicate reliance on water for function has been impossible to rationally engineer – until now.
A team of scientists led by Patrick Barth at EPFL have developed advanced computational tools that aim to shift the scales of GPCRs water-mediated interactions to design new membrane receptors that outperform their natural counterparts. Their work, now published in Nature Chemistry, could lead to better medicines and new tools in synthetic biology.
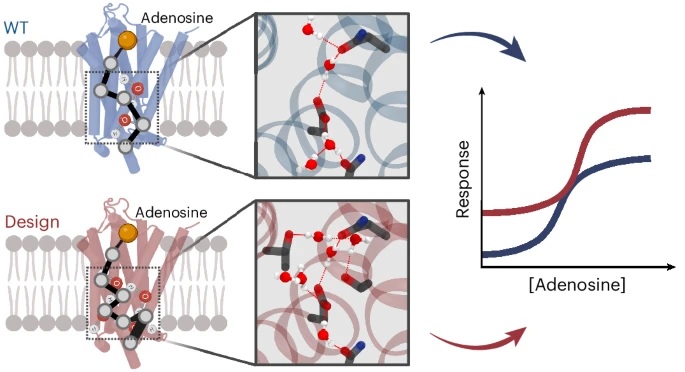
At the heart of the effort is a computational design tool called SPaDES. The researchers used it to create synthetic GPCRs. Starting with the adenosine A2A receptor as a template. they focused on modifying its “communication hubs,” key interaction sites between water molecules and amino acids. These hubs act like switchboards, relaying information throughout the protein. By designing networks that optimize water-mediated connections, the team created 14 new receptor variants.
The SPaDES software allowed them to simulate how these changes would affect the receptors’ shapes and functions in different critical states. After computational screening, the team then synthesized the most promising receptors and tested their activities in cells.
What they found was remarkable: the density of water-mediated interactions turned out to be a key determinant of receptor activity. Receptors with more of these interactions showed higher stability and signaling efficiency. The most promising design, called Hyd_high7, even adopted an unexpected and unforeseen shape, validating the design models.
The 14 new receptors outperformed their natural counterparts in several ways, including their ability to remain stable at high temperatures and their enhanced ability to bind signaling molecules. These qualities make them not only functionally superior but also more robust for use in drug discovery and synthetic biology.
The work holds enormous potential for medicine and biotechnology. By enabling the precise engineering of membrane receptors, the new method could lead to better-targeted therapies for diseases like cancer and neurological disorders. Beyond medicine, these synthetic receptors could be used in biosensors or other tools for detecting environmental changes.\
The findings also challenge long-held assumptions about how GPCRs work, revealing an unexpected flexibility in their water-mediated interaction networks. This opens new avenues for exploring an untapped potential of these proteins in both nature and the lab.






