NOIDA: The drugs control department of Gautam Budh Nagar district has registered a case against two Himachal Pradesh-located manufacturers/producers for allegedly making and selling “unfit” antibiotic medicines, officials aware of the matter informed on Thursday.
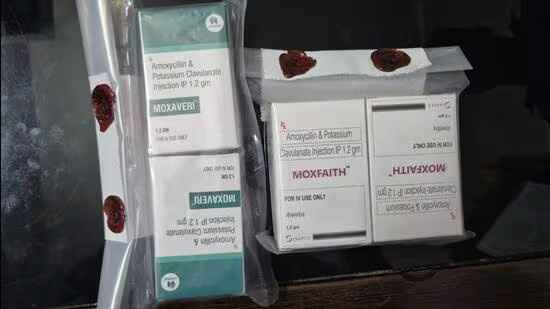
A recent inspection conducted across medical stores operating in Gautam Budh Nagar revealed that the two medicines “moxfaith” and “moxaveri” were “sub-standard” or “adulterated”, and unsafe for consumption, they said.
A case has been registered against the erring manufacturers and further legal action is underway, informed officials.
Inspections were conducted following directions of the Food Safety and Drug Administration Department and Gautam Budh Nagar district magistrate Manish Kumar Verma recently to ensure drugs being sold at medical stores and pharmacies are up to required safety standards.
The medicines found unfit during inspections were confiscated by the department, said district drug inspector Vaibhav Babbar.
“Raids were conducted at several pharmacies and medical stores operating in different parts of the district recently. It came to light that two antibiotic medicines that were being manufactured by Himachal Pradesh-based production units were ‘sub-standard’ and ‘unfit’ for consumption,” said Babbar.
According to the information from the district drug control department, the two firms based in Himachal Pradesh have been booked under relevant Sections of the Drugs and Cosmetics Act, 1940.
“Samples of the two medicines were collected and sent for examination which revealed them to be of ‘substandard’ quality,” Babbar said.
“Manufacturing and selling drugs ‘unfit’ for consumption is a violation of Section 18 (prohibition of manufacture and sale of certain drugs and cosmetics) and is a punishable offence under Section 27 of the Drugs and Cosmetics Act, 1940”, added the district drug inspector, he added.
Earlier this year, the regional ayurvedic and unani office, Gautam Budh Nagar, had banned the production, supply and sale of several ayurveda pills and powders after being allegedly found to be “spurious” and “adulterated”, having composition of allopathic drugs including Prednisolone, Diclofenac, Betamethasone, among others.






