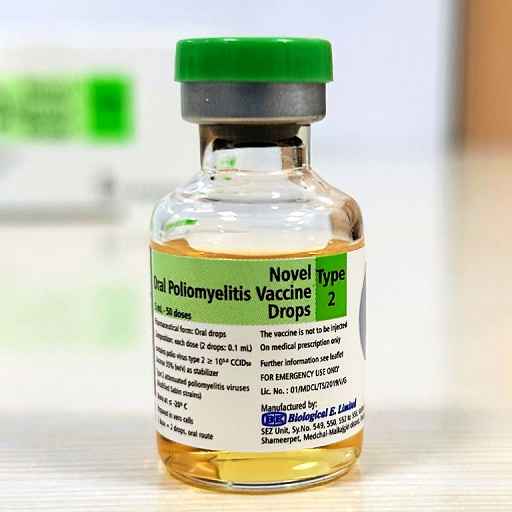
Biological E Receives WHO PQ For Novel Oral Polio Vaccine
Hyderabad: In a monumental stride towards eradicating polio worldwide, Biological E. Limited (BE), a Hyderabad-based vaccine and pharmaceutical company today announced that the World Health Organisation (WHO) has granted Pre-qualification (PQ)…
Pune: Two-Day Training On Medicinal Plants At SPPU Attracts 25 Trainees
Pune: A two-day training on “Processing and Value Addition of Medicinal Plants” was organised on July 27 and 28 at the National Medicinal Plants Board, Ministry of AYUSH sponsored West Regional…
Drug Safety Alert: IPC Flags ADR Linked To Vancomycin
New Delhi: The Indian Pharmacopoeia Commission (IPC), through its recently issued drug safety alert for the month of July, has revealed that Vancomycin, a tricyclic glycopeptide antibiotic, is linked with adverse drug reactions…
Cipla To Commence Supplies To US From China Plant Later This Year
New Delhi: Drug major Cipla expects to commence supplies to the US market from its China facility in the second half of the current fiscal after getting approval from the…
Industry Urges DoP To Implement Prospective Batch Pricing To Avoid DPCO Based Litigations
Mumbai: Industry associations have urged the Department of Pharmaceuticals (DoP) to implement prospective batch pricing to avoid Drug Prices Control Order (DPCO) based litigations. Currently, once ceiling prices are notified,…
Mankind Pharma Acquires Bharat Serums And Vaccines For Rs 13,600 Crore
Mumbai: Mankind Pharma on Thursday said it has entered into a definitive agreement to acquire a 100 per cent stake in Bharat Serums and Vaccines (BSV) from private equity firm Advent International for…
Australian Mayne Pharma Sues Sun Pharma Over Patent Infringement
Canberra: Mayne Pharma has filed a lawsuit against Sun Pharma over infringements of patents related to a certain product used for menopause-related vaginal pain, the Australian drugmaker said on Thursday.…
US FDA Finds Data Integrity, Sterility Problems At Brassica Pharma
Maryland: The US Food and Drug Administration (FDA) has warned Brassica Pharma for numerous good manufacturing practice (GMP) violations, including multiple instances of employees falsifying sterility and environmental monitoring data and…
Indian National Charged With Selling Counterfeit Cancer Drugs
HOUSTON: A federal grand jury has returned an indictment charging an Indian national with selling and shipping tens of thousands of dollars in counterfeit oncology pharmaceuticals into the United States,…
BIS Sanctioned 82 Medical Device Projects To Develop Standards
New Delhi: The Bureau of Indian Standards (BIS) has sanctioned 82 Research & Development (R&D) projects to faculty members of various prominent technical institutes, including IITs, NITs, and other experts, to…