Indore Lab Under Scrutiny: Suspected of Issuing Manipulated Drug Reports Without Proper Testing,pious labs
February – In a shocking revelation, the Pious Lab located in the Rangwasa area of Indore has come under intense scrutiny following a surprise raid by the Food and Drug…
Centre flags serious lapses in state’s blood banks
Jaipur: Union health ministry has flagged “serious lapses” in Rajasthan’s blood bank management after inspections found shortcomings including inadequate record-keeping, lack of proper ELISA testing, and failures to communicate HIV-positive…
Allahabad HC refuses to quash case against pharma company linked to cough syrup deaths in Uzbekistan
Allahabad: The Allahabad High Court recently declined to quash proceedings under the Drugs and Cosmetics Act against the directors of a company accused of manufacturing sub-standard cough syrup that caused…
IPC registers 44 small-scale medtech firms under ADRMS
Mumbai: The Indian Pharmacopoeia Commission (IPC) has registered 44 small-scale medtech firms under ADR Monitoring System (ADRMS) to help them report medical devices related serious adverse events (SAEs) for patient and…
Punjab-Based Company Booked For Producing ‘Substandard’ Antibiotic
Noida: The drugs control department of Gautam Budh Nagar on Tuesday booked a Mohali-based pharmaceutical company for allegedly producing “substandard” quality antibiotics, which were found being sold in Gautam Budh…
60 Small Drug Makers Join Govt Scheme To Modernise Pharma Manufacturing Units
New Delhi: Sixty small drug manufacturers have enrolled in a government scheme aimed at modernising their units to align with global good manufacturing practices (GMP), according to a report by the…
Lilly Received Marketing Authorization For Tirzepatide In India
New Delhi: The popular weight loss and diabetes drug Tirzepatide — marketed as Mounjaro and Zepbound in the US — is likely to hit the markets in India though there has…
DCA Seizes Overpriced, Misleading Claim Medicine
Hyderabad: Officials from the Telangana Drugs Control Administration (DCA) in separate raids in Sangareddy and Suryapet districts seized overpriced drugs and also medicines with misleading advertisements. During a special drive, DCA…
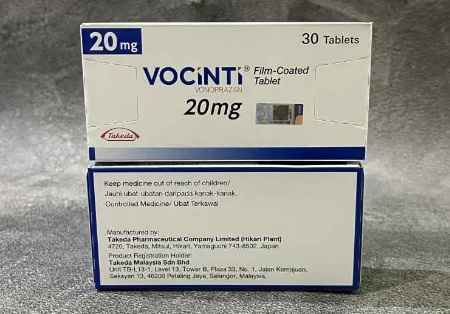
Sun Pharma Inks Non-Exclusive License Agreement With Takeda To Introduce Vonoprazan Tablets In India
New Delhi: Sun Pharmaceutical Industries on Friday said it has inked a licensing pact with Takeda Pharmaceutical Company to commercialise a novel gastrointestinal drug in India. The company has entered…
DCGI Mandates Adherence To BIS Standards For Medical Devices
New Delhi: The Drugs Controller General of India (DCGI), Rajeev Raghuvanshi, has mandated that medical device manufacturers and in-vitro diagnostic (IVD) testing laboratories comply with the Bureau of Indian Standards (BIS)…