Non-Vegetarian Ingredient In Herbal Tooth Powder, Delhi HC Issues Notice To Patanjali Ayurveda And Baba Ramdev
New Delhi: Patanjali Ayurved Limited, led by yoga guru Ramdev, is once again under legal scrutiny as the Delhi high court has demanded responses from the Centre, the Food Safety and…
Presence Of Microplastics In Salt Sugar Brands NGT Issues Notice To Authorities
New Delhi: The National Green Tribunal has called on the Indian Council of Medical Research (ICMR) and the Food Safety and Standards Authority of India (FSSAI) to address the issue of…
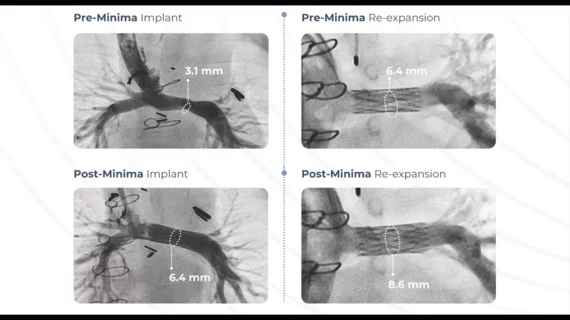
Renata Medical Receives FDA Approval For The Minima Growth Stent
Maryland: The FDA has approved a new type of stent designed specifically for infants and young children—including neonates born with congenital heart defects—that’s designed to expand in size as the child…
Centre Released Around Rs. 700 Crore For Strengthening Of State Drug Regulatory Mechanism
New Delhi: The Central government has released more than 80 per cent of the funds allocated as Central share for the strengthening of drug regulatory system across the States including…
Revised GMP Norms For Small Drug Firms To Be Issued Soon
New Delhi: The government will shortly notify a revised version of good manufacturing practices (GMP), at par with the World Health Organization (WHO) standards, for small pharmaceutical companies with an annual…
IIL In Collaboration With CIBA-ICAR To Produce Vaccines For Finfish
Hyderabad: ICAR-Central Institute of Brackishwater Aquaculture (ICAR-CIBA), Chennai signed a partnership agreement with Indian Immunologicals Limited, Hyderabad for the commercial development of CIBA vaccine “Nodavac-R” against viral nervous necrosis in finfishes…
Pharma Firms Move Delhi HC, Seek Quashing Of Notifications Banning 156 FDC
New Delhi: Pharma companies such as Alkem Laboratories and Entod Pharmaceuticals Pvt Ltd and others have moved the Delhi High Court seeking that the government’s recent notification banning 156 fixed-dose combination…
CDSCO Framing Guidelines For Proper Disposal Of Expired And Unused Medicines To Prevent Misuse
New Delhi: The Central Drugs Standard Control Organization (CDSCO), India’s apex drug regulator, is framing guidelines for disposal of expired and unused medicines to prevent their misuse and to curb…
Telangana: DCA Raids Uncover Fake Medicines, Unlicensed Clinics
Hyderabad: The Drugs Control Administration (DCA) of Warangal Zone conducted several raids on Tuesday, August 27 and Wednesday, August 28 that uncovered several violations, like illegal manufacture and sale of drugs…
Bharat Biotech Launches Oral Cholera Vaccine
Hyderabad: Bharat Biotech International Ltd on Tuesday announced the launch of Hillchol, a novel single-strain oral cholera vaccine following a successful phase-III clinical trial which confirmed the safety of the jab.…