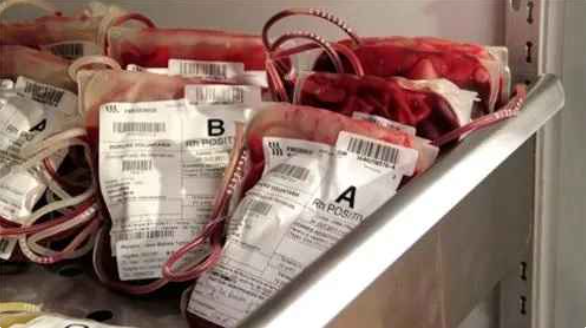
Centre flags serious lapses in state’s blood banks
Jaipur: Union health ministry has flagged “serious lapses” in Rajasthan’s blood bank management after inspections found shortcomings including inadequate record-keeping, lack of proper ELISA testing, and failures to communicate HIV-positive…
IPC registers 44 small-scale medtech firms under ADRMS
Mumbai: The Indian Pharmacopoeia Commission (IPC) has registered 44 small-scale medtech firms under ADR Monitoring System (ADRMS) to help them report medical devices related serious adverse events (SAEs) for patient and…
Wrong medicine without a prescription, 5-month-old infant died: FIR against Medical store owner
Mauganj: In Mauganj, Madhya Pradesh, a First Information Report (FIR) was registered against a medical store owner on Sunday after a 5-month-old infant died from being given the wrong medicine…
CSIR-NIIST Develops Technology To Convert Biomedical Waste Into Soil Additives
THIRUVANANTHAPURAM: CSIR-National Institute for Interdisciplinary Science and Technology (NIIST) in Thiruvananthapuram has developed a technology that converts pathogenic biomedical waste into value-added soil additives that eliminate the need for costly…
60 Small Drug Makers Join Govt Scheme To Modernise Pharma Manufacturing Units
New Delhi: Sixty small drug manufacturers have enrolled in a government scheme aimed at modernising their units to align with global good manufacturing practices (GMP), according to a report by the…
FDCA Raids Surat Firms Selling Fake Skin Products
Ahmedabad: Gujarat FDCA, in its third raid on fake allopathic drugs and cosmetics in the state in the last two months, busted three firms, all linked, in Surat for selling counterfeit…
Lilly Received Marketing Authorization For Tirzepatide In India
New Delhi: The popular weight loss and diabetes drug Tirzepatide — marketed as Mounjaro and Zepbound in the US — is likely to hit the markets in India though there has…
Madras HC Sets Aside Ban On Online Sale Of Medicines
Chennai: In a relief for online pharmacies, including Tata 1mg, Practo, PharmEasy and NetMeds, the Madras High Court has reportedly set aside an earlier order by a single judge bench…
Glenmark Recalls 114 Batches Of Low Potassium Drug In The US Over Potentially Deadly Hyperkalemia Risk
MAHWAH, N.J. : While patients with low potassium often need medication to boost levels of the electrolyte in the body, too much of a good thing can cause serious health concerns.…
DCA Seizes Overpriced, Misleading Claim Medicine
Hyderabad: Officials from the Telangana Drugs Control Administration (DCA) in separate raids in Sangareddy and Suryapet districts seized overpriced drugs and also medicines with misleading advertisements. During a special drive, DCA…